|
You may also be interested in the following articles:
|
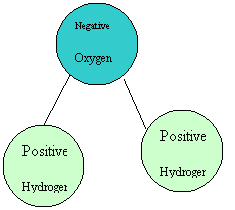
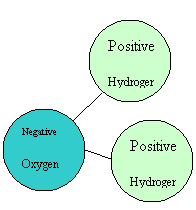
Hydrophilic Substances Barite, hematite, minerals in drilling fluids, some membranes, glucose/sugar and many other substances are known as hydrophilic. In comparison, oils, proteins, colloids, greases and clays are hydrophobic. What does this mean? Does it really matter to non-scientists? Definitions Hydrophilic ~ water loving. Such compounds have an affinity to water and are usually charged or have polar side groups to their structure that will attract water. Hydrophobic ~ water hating. These compounds are repelled by water and are usually neutral (no charge.) In more depth, these terms have much to do with the structure of water itself. Water consists of two hydrogen atoms joined to one oxygen atom, all in a triangular pattern. The oxygen is negatively charged whilst the hydrogen end is positively charged. Thus, water molecules are actually attracted to each other and form hydrogen bonds.
Water Molecule Water Molecule Hydrogen bonds are very important to living things as these bonds allow the strands of DNA to hold together, proteins to form and water to behave as it does. Adding drops of oil or particles of clay or grease to a container of water will not result in a solution as adding sugar particles would. In fact, there will be a ball of oil or clay formed; forming a ball reduces the overall amount of surface area exposed to the water. Although it may appear that the oil is gathering into a drop, the reality is that water is highly attracted to other water molecules and ignores the hydrophobic molecules; the oil molecules are then squeezed together to allow the water to form more hydrogen bonds. Non-polar molecules are neither attracted to nor repelled by each other so the pull between water molecules is much stronger. Hydrophilic molecules attract the water molecules and they blend together, making water a very good solvent in many situations. Uses The hydrophilic properties of certain substances can be of great use in industry, especially in terms of keeping essential areas clean. For instance, a hydrophilic membrane will not be fouled with oil, grease or other hydrophobic substances. The membrane is highly attractive to water so the water molecules will push away other molecules in order to gain access to the membrane. Once formed, hydrogen bonds are quite stable and reluctant to break apart. This keeps contaminants away from the membrane so it remains clean and functioning for longer. Hydrophilic coatings or materials become wet very easily, and maintain the wetness for longer than a hydrophobic equivalent would. Thus, using hydrophilic coatings or materials for tubes and hoses eliminates the need for additional lubricants. This is of particular usefulness where sterility or cross contamination issues may arise. Hydrophilic coatings of rubber can increase the power of plugs and o-rings, etc to stop water escaping to cause leaks. The water is attracted to the coating and less available for seeping through gaps. This is the basis for waterstop and sealant products. On the other hand, hydrophilic grouts are less useful than hydrophobic ones in wet areas. Hydrophobic grouts absorb only enough water to bind the compounds; it is stable once in use. Hydrophilic grouts are prone to lose and attract water as conditions vary and thus contract and expand. This movement can be destructive and absorption of water may add contaminants to the grout; hydrophilic grouts generally donít last as long as their hydrophobic counterparts. Hydrophilic grouts are great for stopping leaks, but not for filling in large voids. Other uses of hydrophilic substances are catheters, contact lens cleaners, gums in frozen dough, waterproofing outside materials (e.g. horse blankets,) surface treatment of wet wipes and nappies, wound dressings and scientific testing for proteins and the like.
Tash Hughes has a science degree and experience in major engineering companies. As well as having written Expressions of Interest, Tash has written analytical and procedural documents. All technical and business writing needs are met by www.wordconstructions.com.au
|